Therefore, stable in ground state On the other hand, a system with (4n)πelectrons is antiaromatic and hence, unstable inDonate here http//wwwaklecturescom/donatephpWebsite video link http//wwwaklecturescom/lecture/thermal42cycloadditionreactionFacebook link https Electrocyclic Reactionswoodward Hoffmann Rule For 4n And (4n 2) Pi Systems this video explains the experimental results of electrocyclic reactions involving 4n and (4n 2) pi systems with the help of woodward hoffmann rule this video dr norris reviews the woodward hoffmann rules for electrocyclic reactions and does some example problems the following
Non Ionic Chemical Reactions
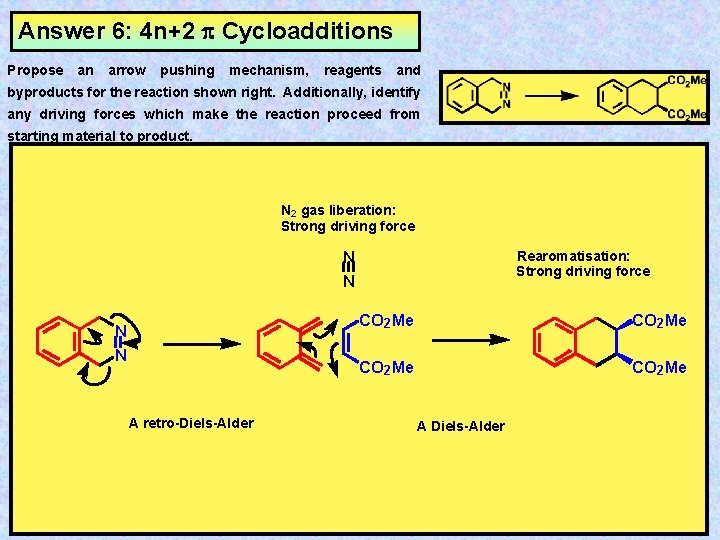
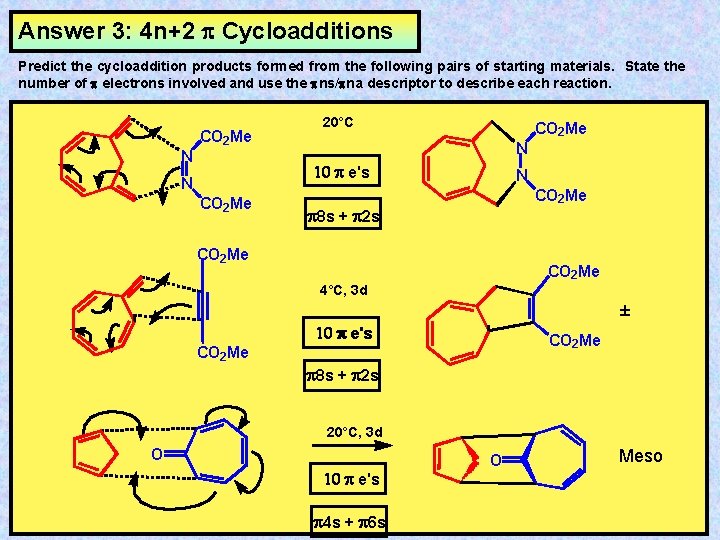
4n+2 cycloaddition reaction
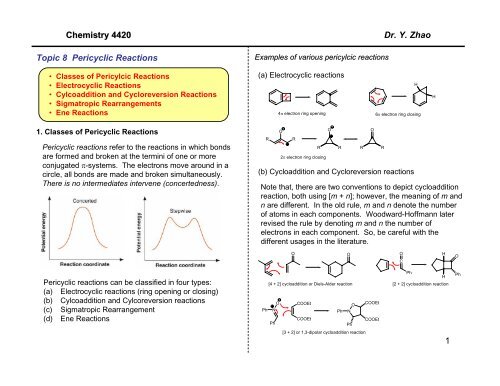
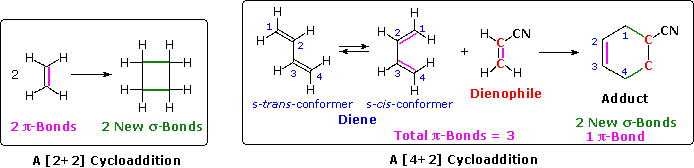
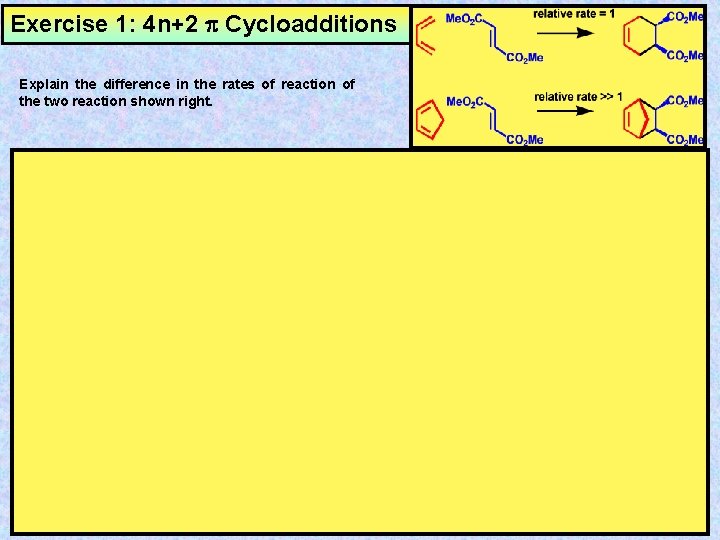
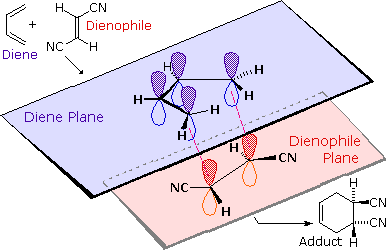
4n+2 cycloaddition reaction-Migration of a σbond;A Cycloaddition is a pericyclic chemical reaction, in which two π bonds are lost and two σ bonds are gained the resulting reaction is a cyclization reaction Cycloadditions are usually described by the backbone size of the participants This would make the DielsAlder a 4 2cycloaddition, and the 1,3dipolar cycloaddition a 3 2cycloaddition



Cycloaddition
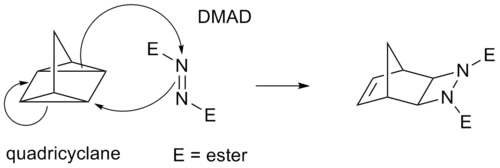
Molecules with Multiple Bonds Incorporated in or Linked to Aromatic SystemsMay be intermolecular or intramolecular 2) Electrocyclic reactions 11 Intramolecular 3) Sigmatropic reactions 0 0 Intramolecular;DMAD is a reagent that reacts readily with dienes Two independent 4n2 electron thermal electrocyclic reactions convert ditropyl into two separate diene units, followed by 4n2 thermal cycloaddition of the DMAD to give B Four different stereoisomers are possible, but the two unsymmetrical forms are ruled out from the nmr evidence
Read "Cycloaddition Reactions Involving 4n Electrons 2 2 Cycloaddition;The DielsAlder Reaction (DAR), reported in 1928 by Otto Diels and Kurt Alder, is a thermal, concerted, suprafacial, 42 cycloaddition The prototypical DAR is illustrated in Fig 6 While 1,3butadiene is a viable diene in the reaction, ethylene itself is a poor dienophile (dienophobe?)See the answer See the answer See the answer done loading Show transcribed image text Expert Answer
Cycloaddition Reactions Involving 4n Electrons 2 2 Cycloaddition;One of the most famous cycloaddition reactions is the Diels–Alder reaction (D–A), which is a 42cycloaddition reaction between a diene (4πcomponent, eg, 1,3butadiene) and a dienophile (2πcomponent, eg, ethylene) D–A reaction was first discovered by Diels and Alder in 1928 10 and in 1950 they together received the Nobel PrizeCONTROLS Click the structures and reaction arrows in sequence to view the 3D models and animations respectively 22 Ketene Cycloadditions are one of the few 22 thermal cycloadditions allowed The 22 Ketene addition is allowed as the transition state is stablised by the pi orbital of the Carbonyl stablising the electron difficent



1



A Disrotatory 4n 2 Electron Anti Aromatic Mobius Transition State For A Thermal Electrocyclic Reaction Henry Rzepa S Blog
32 cycloaddition (54 cycloaddition 42 cycloaddition 4n2 cycloaddition This problem has been solved!Fax 007 (347 2) 35 6066 The DielsAlder reaction of levoglucosenone with pipe~,lene catalyzed by ZnCI 2 proceeds regio and stereoselectively to give the sincisadduct 3;The stereochemical outcome of a reaction indicates whether a (ss) or a (sa) approach is involved (ss) Cycloadditions preserve the stereochemistry of the starting materials For example, cis substituted alkenes produce syn products in 24 cycloadditions At first it cannot be determined how the substituents of alkene and diene relate to



Ochem



Pericyclic Reactions Electrocyclisation Sigmatropic Cycloadditions Cheletropic Reactions Frontier
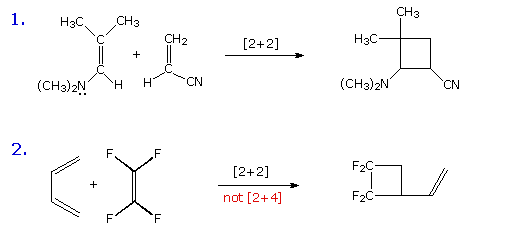
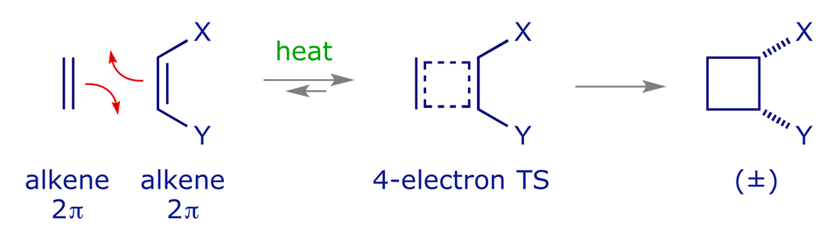
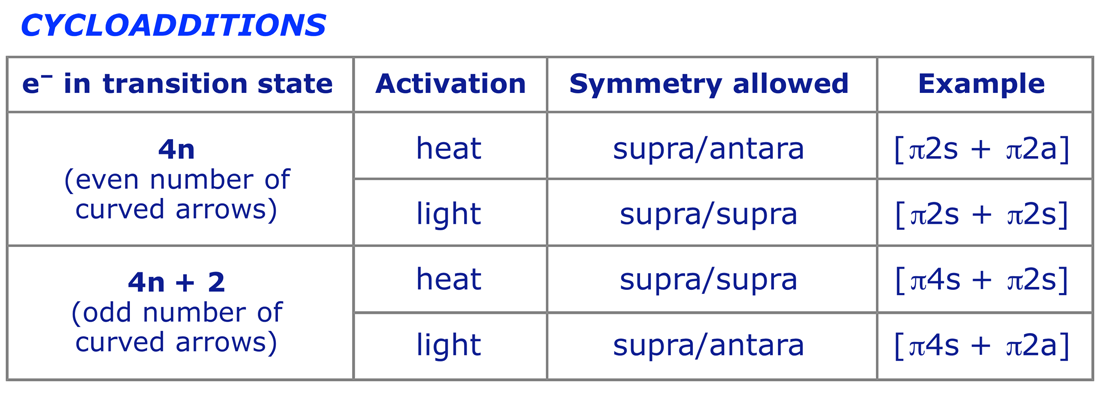
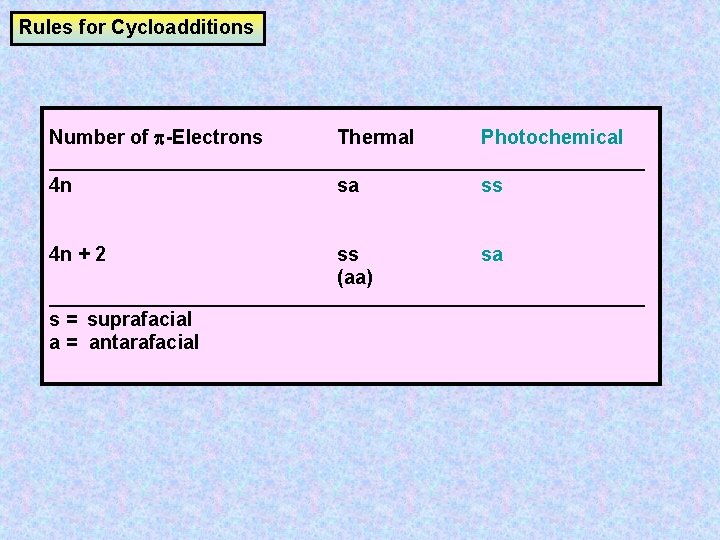
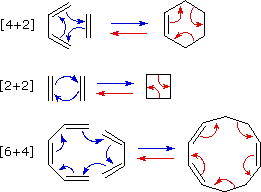
The 2 2cycloaddition of a sulfene with an imine, also called the sulfaStaudinger cycloaddition, is a common method to prepare βsultamsThis reaction offers a straightforward access to the two diastereoisomers, the cis (69) and the trans βsultam (70), depending on the conditions and the substitution pattern around the sulfonyl chloride and the imine (Scheme 35)Cycloaddition reactions 2 2 A cyclic product is formed;These reactions can take place both photochemically and thermally, this generally depends on the number of electrons All suprafacialsuprafacial cycloadditions with 4n electrons (ie 2 2, 4 4) always run photochemically, all with 4n 2 electrons 4 2 always run thermally



1 Chemistry 44 Dr Y Zhao Topic 8 Pericyclic Reactions




What Is A Cycloaddition Reaction Quora
As you know, cycloaddition reactions which (4n)π electrons are involved in such as 22cycloaddition are forbidden in normal organic reactions, but transition metal catalyzed 22cycloaddition reactions are allowed I think the d orbitals of transition metals play important roles in the reactions, but I cannot understand how they work The vast majority of 22 photocycloaddition reactions involves enone–alkene cycloaddition which is conveniently achieved through direct excitation or sensitization by UV irradiation Substantial progress has also been made in 22 cycloaddition between two unactivated alkenes using transition metal salts especially copper(I) saltsThe first step in the synthesis of pentacyclo5400 2,60 3,100 5,9 undecane8,11dione, a pentacyclic cage compound, is a DielsAlder reaction, which is an example of a thermal 42cycloaddition The reaction involves the addition of a 4pcomponent (the diene) to a 2pcomponent (the dienophile) in a 6pelectron process, which is "allowed




Duality Of Orbital Symmetry Allowed Transition States For Thermal Sigmatropic Hydrogen Shifts In Transition Metal Compounds Mauksch 16 Chemistry A European Journal Wiley Online Library




Cycloaddition An Overview Sciencedirect Topics
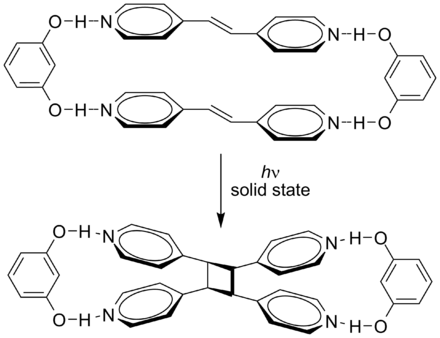
Photochemical Energy Storage Systems Based on Reversible Valence Photoisomerization, ChemInform" on DeepDyve, the largest online rental service for scholarly research with thousands of academic publications available at your fingertipsThe thermal reaction yields an epimeric mixture of compound 3 with sintransadduct 4 in the ratio of 4 I Key words levoglucosenone, piperylene, cycloaddition, regioRearrangement of πelectrons 4) Group transfer reactions 11 Intermolecular transfer of a group;



1



Other Cycloadditions
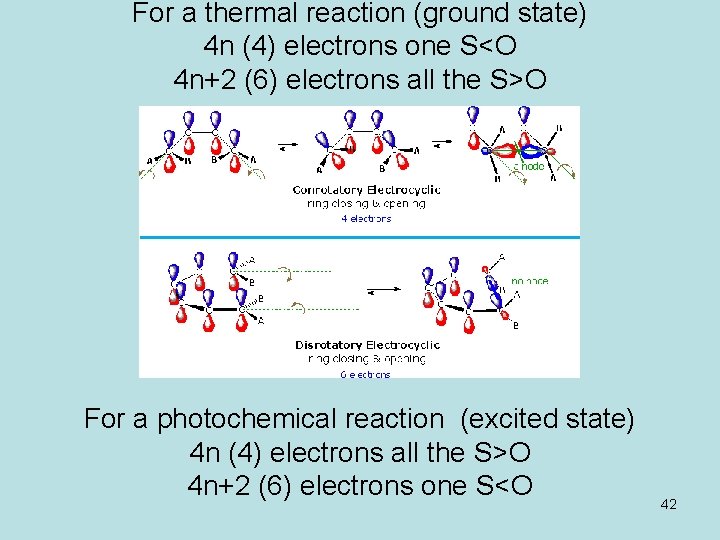
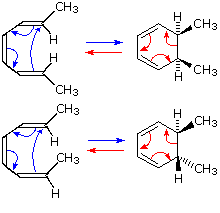
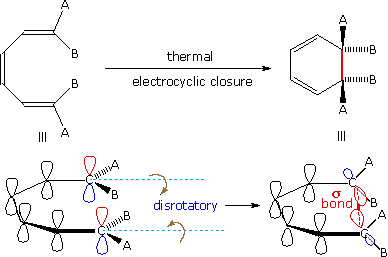
A cycloaddition is a chemical reaction between reactants with double bonds that get replaced by a ring structure It is a pericyclic chemical reaction where "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity" It is a cyclization reaction it makes new ring of atoms Cycloadditions are namedA 4n electron electrocyclic reaction achieves constructive HOMO orbital overlap if it is conrotatory, while a 4n2 electrocyclic reactions achieves constructive overlap if it is disrotatory Cycloaddition reaction Selection rules pq thermal light 4n p sqaor paqspsqsor paqa 35 1,3Dipolar cycloaddition reactions A similar cycloaddition of nitrile oxides provides a method for the synthesis of 3hydroxy ketones, all these reactions involve 4n2 electrons and are suprafacial ON Ph Ph Ph HONHPh Ph Ph Zn(s) (reducing agent) ON Ph Ph Ph O N Ph Ph Ph 32 cycloaddition The cycloaddition of nitrones to alkenes (below




Cycloadditions Pericyclic Reaction Notes Docsity



16 6 The Diels Alder 4 2 Cycloaddition Reaction Chemistry Libretexts
The reaction modes occurring between the azo dyes and 1O2 were the ene and/or 2 2 cycloaddition reactions, and they occurred at the double bondsMigration of a σbond from one molecule to another; ChemInform Abstract Cycloaddition Reactions Involving 4n Electrons (2 2) Cycloaddition Photochemical Energy Storage Systems Based on Reversible Valence Photoisomerization JONES, G II ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals



Woodward Hoffmann Rules Chemistry Libretexts



Pericyclic Problems Answers
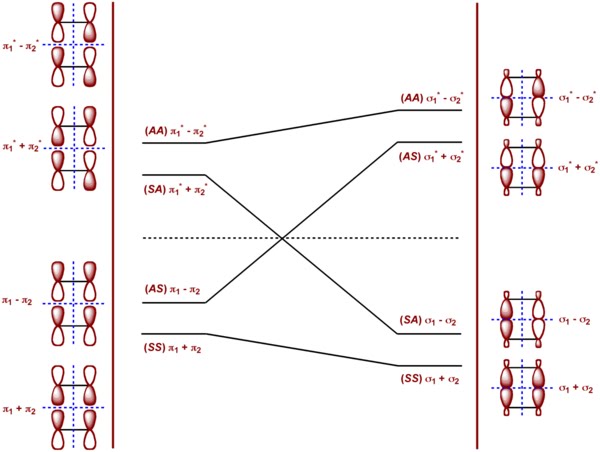
Diene dienophile HOMO π2 LUMO π* 3 π* 4 π1 π1bonding HOMO π∗2antibondingHigh on cycloaddi(c)tions The impact of the WoodwardHoffmann 4n2 rule for thermally allowed cycloaddition reactions and the challenges associated with moving from six to ten electrons are discussed in this Essay Transcribed image text 41 THIS REACTIONIS CLASSIFIED AS (1 نقطة) н H Photochemical 2 2 cycloaddition reaction THERMAL 2 2 cycloaddition reaction CYCLOADDITION, ANTARAFACIAL THERMAL 2 4 cycloaddition reaction * THIS ORBITAL MOTION IS (1) (1 نقطة) Clockwise Clockwise CONROTATORY DISROTATORY SUPRAFACIAL
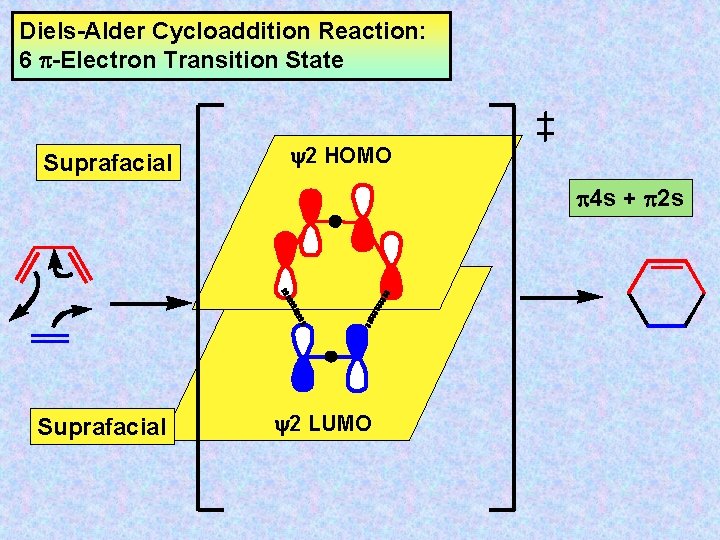



Cycloaddition Reactions Cycloaddition Reactions Are Intermolecular Pericyclic Processes



Electrocyclic Reactions Ppt
Cycloadditions DielsAlder reaction The DielsAlder reaction is a 42cycloaddition between a conjugated diene and an alkene (dienophile) to form a cyclohexene system As all pericyclic reactions the DielsAlder reaction proceeds in a single step Two new σbonds are formed at the same time during a DielsAlder reactionConclusion about pericyclic reactions as above two methods without taking into account symmetry of molecular orbitals According to Huckel's rule of aromaticity a planer conjugated system with (4n 2)πelectrons is aromatic;An Allowed 42 Cycloaddition!



Pericyclic Reactions Cycloadditions And Diels Alder Reaction Chemgapedia



The Stereochemistry Of 8 2 Pericyclic Cycloadditions Henry Rzepa S Blog
Similarly the DielsAlder reaction (Section 21) is a π 2s π 2s cycloaddition, as both of the π components react suprafacially in that process These descriptors of facial reaction mode are important, as they are required in defining the selection rules for pericyclic reactions 3 Thermal concerted 2 π 2 π cycloadditions 22 photocycloaddition 22 Photocycloaddition is the combination of an excited state enone with an alkene to produce a cyclobutane Although the photochemical concerted 22 cycloaddition is allowed, the reaction between enones and alkenes is stepwise and involves discrete diradical intermediatesSymmetryallowed 43 cycloaddition is an attractive method for the formation of historically difficulttoaccess sevenmembered rings Neutral dienes and cationic allyl systems (most commonly oxyallyl cations) may react in a concerted or stepwise fashion to give sevenmembered rings A number of dienes have been employed in the reaction



Cycloaddition Wikiwand



Other Cycloadditions
22 cycloaddition reaction Thermal reaction F or instance, Always HOMO of one component overlap with LUMO of other component to get stable product For example, Results In 4n system, when the mode of reaction is thermal, then supraantara overlap is symmetry forbidden and it is difficult practically but theoratically it is possible Mild thermolysis of tertbutyl alkynyl ethers furnishes aldoketenes, which undergo facile 22 cycloaddition reactions with pendant di and trisubstituted alkenesA wide variety of cisfused cyclobutanones are produced in moderate to high diastereoselectivity and good to excellent yields by this method, and free hydroxyl groups are tolerated in the eneynol etherQuestion What type of reaction is the DielsAlder cycloaddition?



Cycloaddition Reaction Orbital Symmetry



Examples Of Cycloaddition Elimination Reactions
The DielsAlder reaction (a (42)cycloaddition) is π 4 s π 2 s The 1,3dipolar cycloaddition of ozone and an olefin in the first step of ozonolysis (a (32)cycloaddition) is π 4 s π 2 s The cheletropic addition of sulfur dioxide to 1,3butadiene (a (41)cheletropic addition) is ω 0 a π 4 s ω 2 s π 4 sAnswer Furan is aromatic because one of the lone pairs of electrons on the oxygen atom is delocalized into the ring, creating a 4n 2 aromatic system similar to benzene Because of the aromaticity, the molecule is flat and lacks discrete double bonds The other lone pair ofCycloaddition Reactions An assortment of thermal and photochemical cycloaddition reactions are shown here The photochemical reactions are noted by a hν over the arrow The reader should inspect each reaction to determine whether it has a 4n or a 4n2 electron transition state, and deduce the expected stereochemistry



Cycloaddition Wikipedia
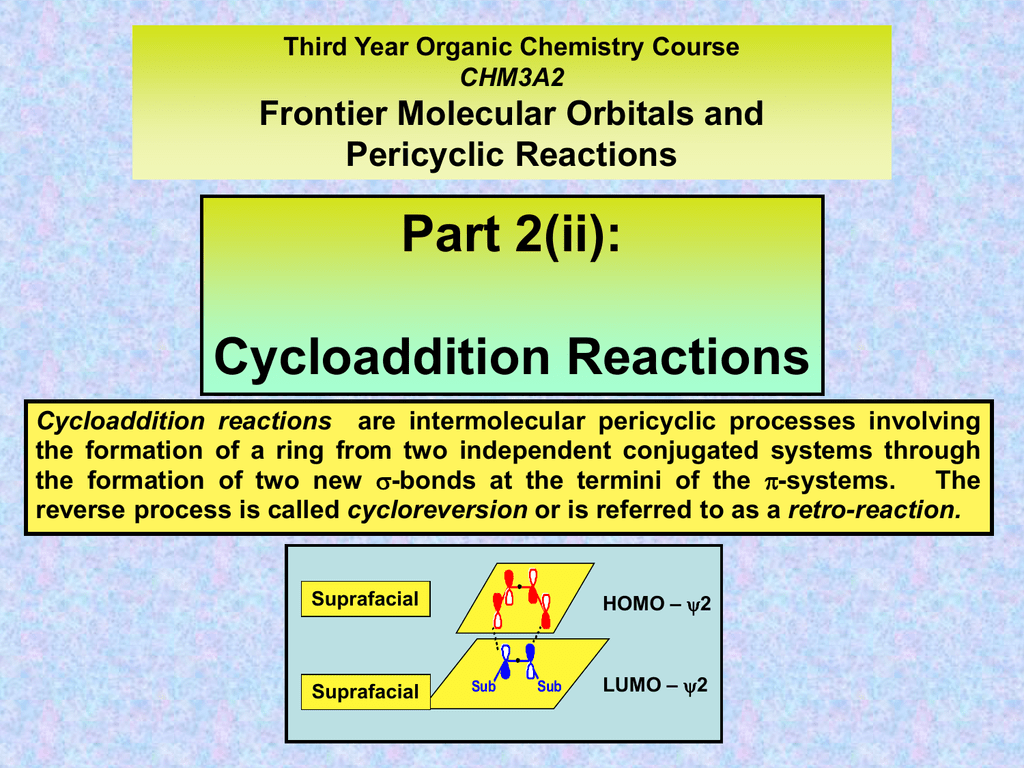



Cycloaddition Reactions
Frontier Molecular Orbital Theory for Cycloaddition Reactions Frontier Molecular Orbital Theory (FMO Theory) Number of Pi Electrons Thermal Photochemical 4n 2 (Odd # of Pairs) SupraSupra Allowed SupraAntara ForbiddenThe stereochemistry of pericyclic cycloadditions Reading Mode Steve Bachrach has blogged on the reaction shown below If it were a pericyclic cycloaddition, both new bonds would form simultaneously, as shown with the indicated arrow pushing Ten electrons would be involved, and in theory, the transition state would have 4n2 aromaticityA cheletropic cycloaddition is a reaction that occurs in which both new bonds are formed to the same atom on one of the reactants The classic example of
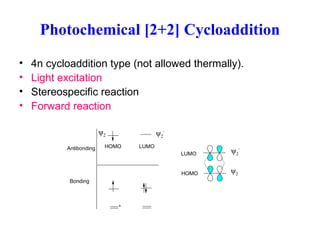



Pericyclic Reaction Ii Pp



Pericyclic Reaction Selection Rules
The most common cycloaddition reaction is the 4 π 2 π cyclization known as the DielsAlder reaction In DielsAlder terminology the two reactants are referred to as the diene and the dienophile The following diagram shows two examples of 4 π 2 π cycloaddition, and in the second equation a subsequent light induced 2 π 2 π cycloaddition In each case the dieneThe DielsAlder reaction is the best known of the cycloaddition reactions It is formally a 42 cycloaddition and can be represented as follows It represents a sixelectron 4 2cycloaddition between a conjugated diene (1,3diene) and the πbond of a substituted alkene (dienophile) to provide a substituted cyclohexeneTion processes Cycloaddition reactions, in particular, are highly valued for their synthetic utility This onestep process represents a facile approach to construct a variety of ring types and increase molecular complexity1 Both (43)and (32)cycloaddition modes of oxyallyl cations are known under thermal conditions and have been investigat




Woodward Hoffmann Rules Wikipedia




Redox Catalysis Cycloaddition Diels Alder Reaction Tris Bipyridine Ruthenium Ii Chloride Diagram Png Pngegg
Cycloaddition Objective/ Theory A cycloaddition is a pericyclic chemical reaction, in which two pi bonds are lost and two sigma bonds are gained Cycloaddition of (4n2) type is concerted and with our intermediates thermally allowed, by means that the reaction proceeds in one step when the constituents are heated properly



Cycloaddition
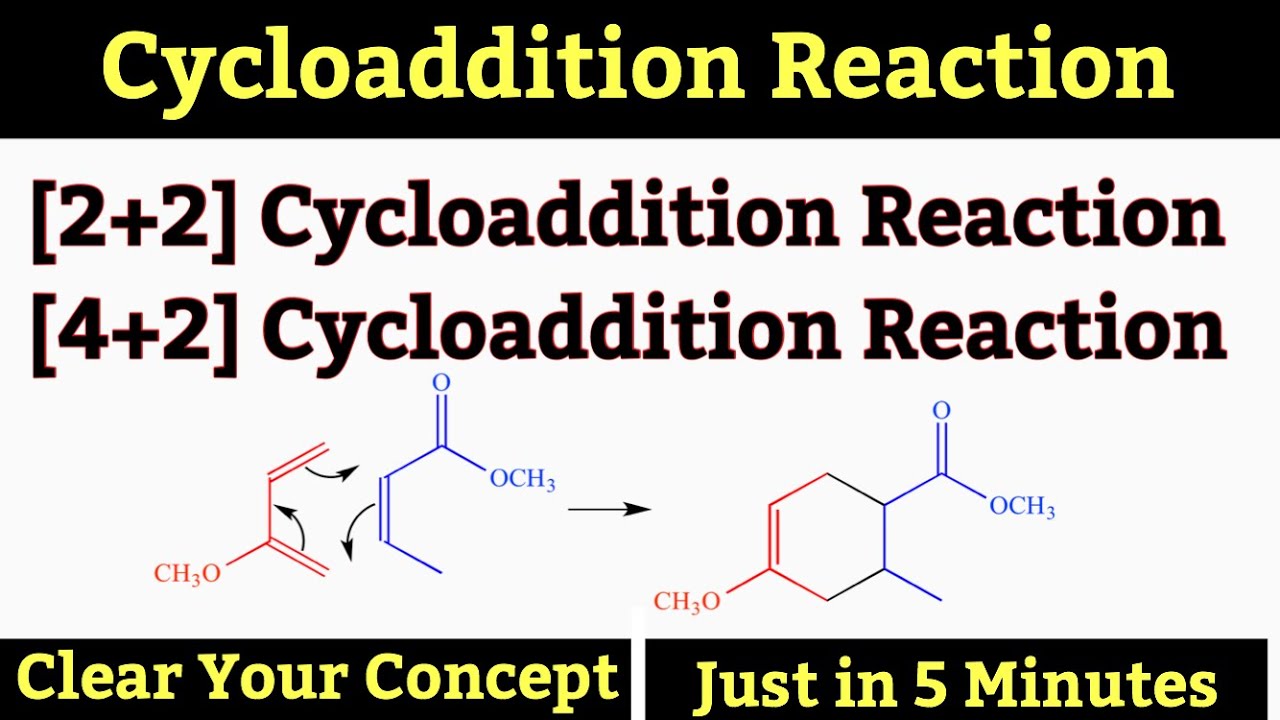



2 2 And 4 2 Cycloaddition Cycloaddition Reaction With Mechanism Organic Chemistry Chemistry Youtube
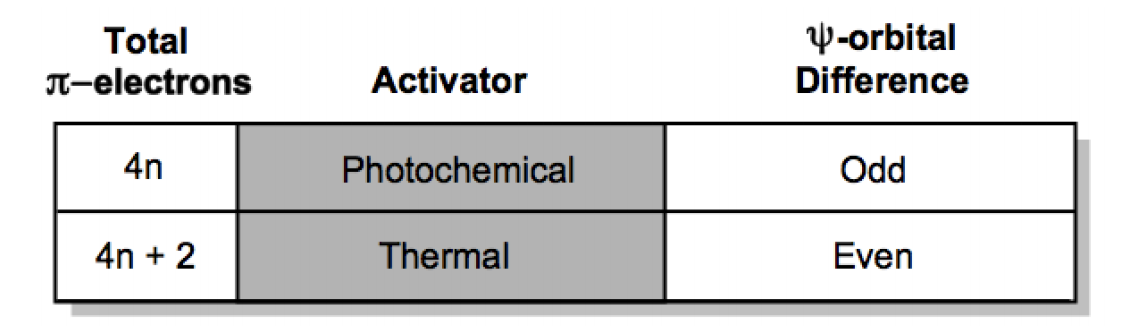



Photochemical Cycloaddition Reactions Organic Chemistry Video Clutch Prep
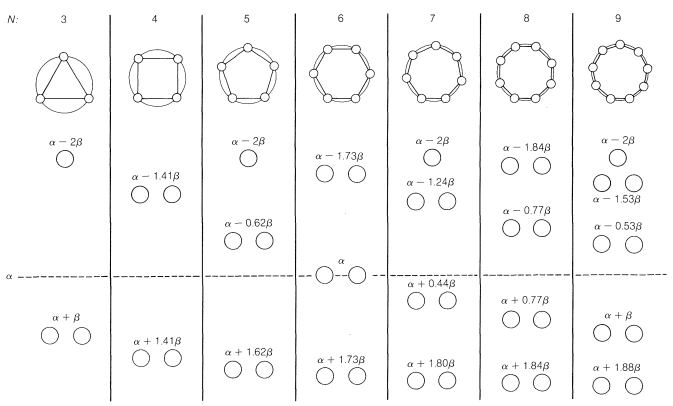



21 11 Pericyclic Reactions Chemistry Libretexts



Correlation Diagram For 4 2 Cycloaddition Reaction Tricky Way Explanation Lecture Youtube
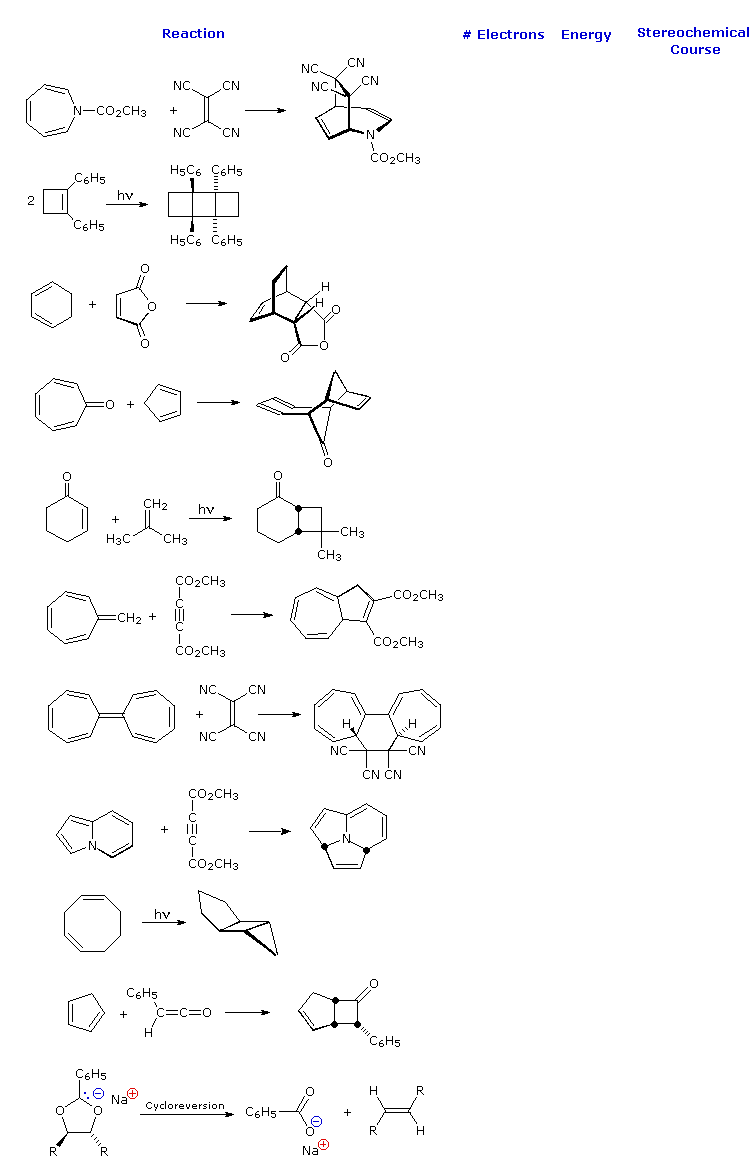



Pericyclic Examples




Overview Pages Perycyclic Book Pages 1 15 Flip Pdf Download Fliphtml5



Cycloaddition Reaction Orbital Symmetry




Overview Pages Perycyclic Book Pages 1 15 Flip Pdf Download Fliphtml5
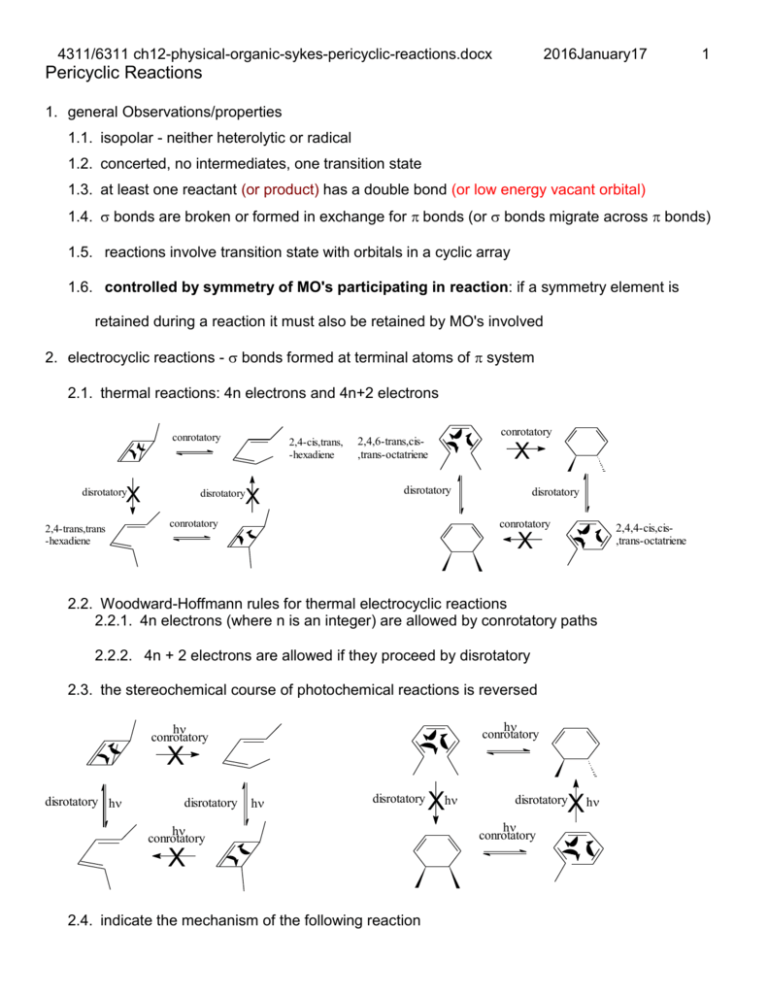



Pericyclic Reactions



Principal Categories Of Organic Pericyclic Reactions



Pericyclic Reactions Cycloadditions And Diels Alder Reaction Chemgapedia



Examples Of Cycloaddition Elimination Reactions



Cycloaddition Reactions Cycloaddition Reactions Are Intermolecular Pericyclic Processes



Cycloaddition



Pericyclic Reactions Cycloadditions And Diels Alder Reaction Chemgapedia



Non Ionic Chemical Reactions



Electrocyclic Reactions Woodward Hoffmann Rule For 4n And 4n 2 Pi Systems Youtube




Electrocyclic Reactions Woodward Hoffmann Rule For 4n And 4n 2 Pi Systems Youtube



Cycloaddition Reactions Cycloaddition Reactions Are Intermolecular Pericyclic Processes




Types Of Pericyclic Reactions



Cycloaddition Reactions Cycloaddition Reactions Are Intermolecular Pericyclic Processes




Organic Chemistry Application Of The Woodward Hoffman Rules To A 14 2 Cycloaddition Chemistry Stack Exchange



Non Ionic Chemical Reactions




Learn About Cycloaddition Reaction Chegg Com



Chemistry 125 Lecture 55 February 24 10 4n 2 Aromaticity Cycloaddition Electrocyclic Reactions This For Copyright Notice See Final Page Of This File Ppt Download



Third Year Organic Chemistry Course Chm 3 A




Cycloaddition Reactions Why Is It So Challenging To Move From Six To Ten Electrons Palazzo 17 Angewandte Chemie International Edition Wiley Online Library
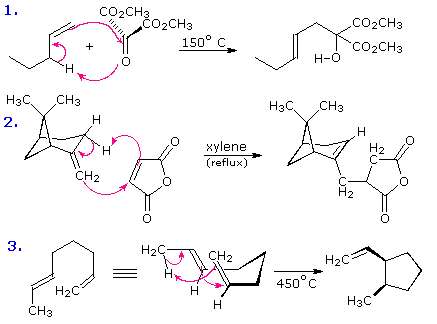



Non Ionic Chemical Reactions



Ochem
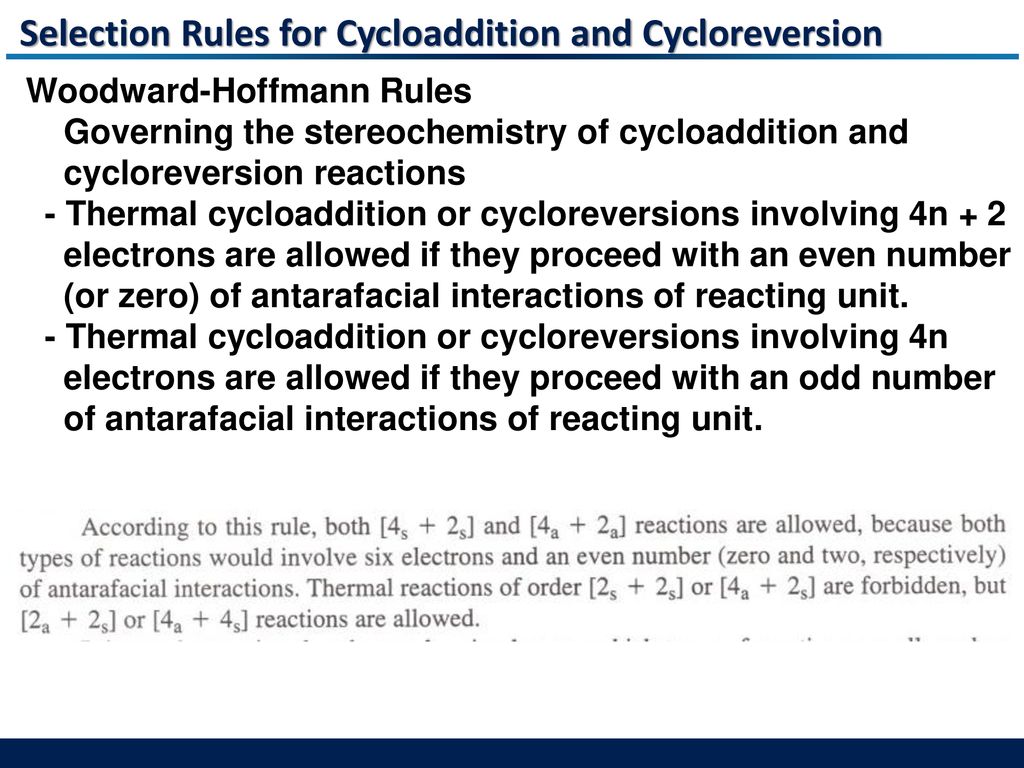



3 Cycloaddition And Cycloreversion Reactions Ppt Download




Cycloaddition Wikiwand



Pericyclic Reactions Cycloadditions And Diels Alder Reaction Chemgapedia




Cycloaddition An Overview Sciencedirect Topics
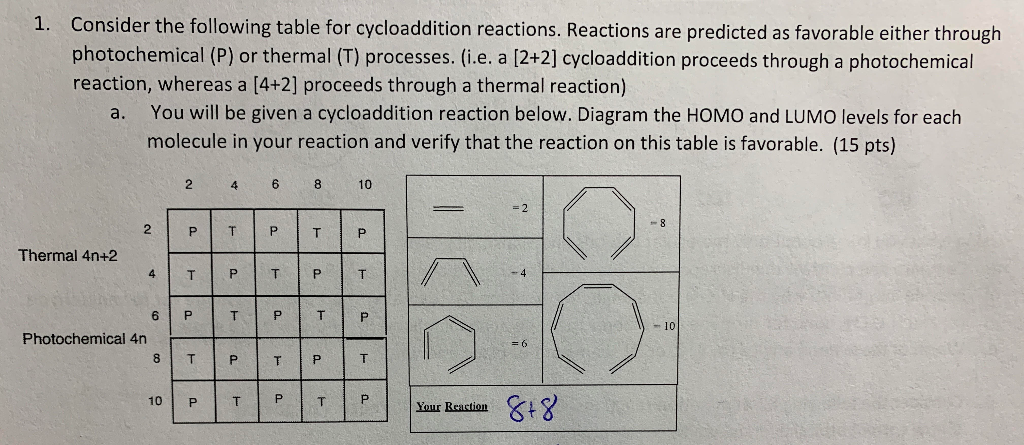



Solved 1 Consider The Following Table For Cycloaddition Chegg Com
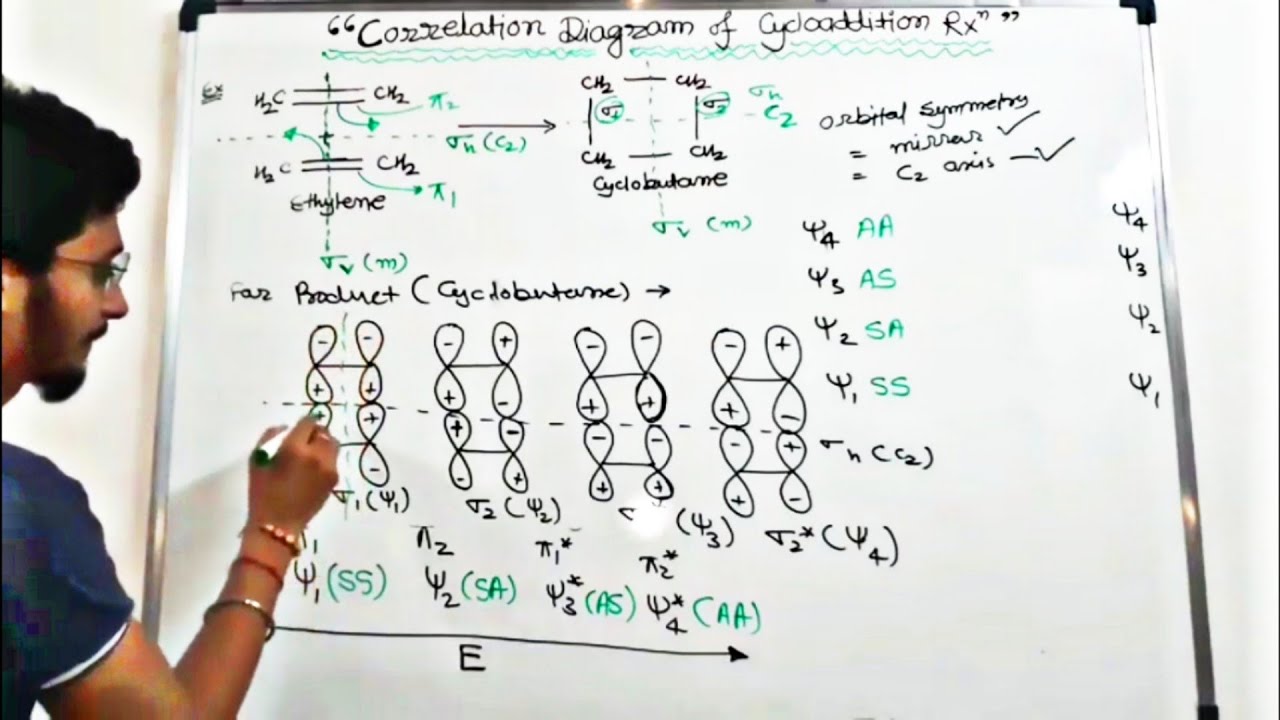



Correlation Diagram For 2 2 Cycloaddition Reaction Tricky Easy Youtube
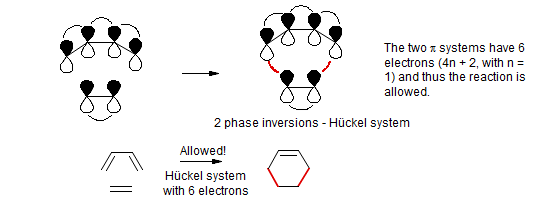



Pericyclic Reactions The Dewar Zimmerman Moebius Huckel Approach



1




Woodward Hoffmann Rules Wikiwand



A Disrotatory 4n 2 Electron Anti Aromatic Mobius Transition State For A Thermal Electrocyclic Reaction Henry Rzepa S Blog




Non Ionic Chemical Reactions
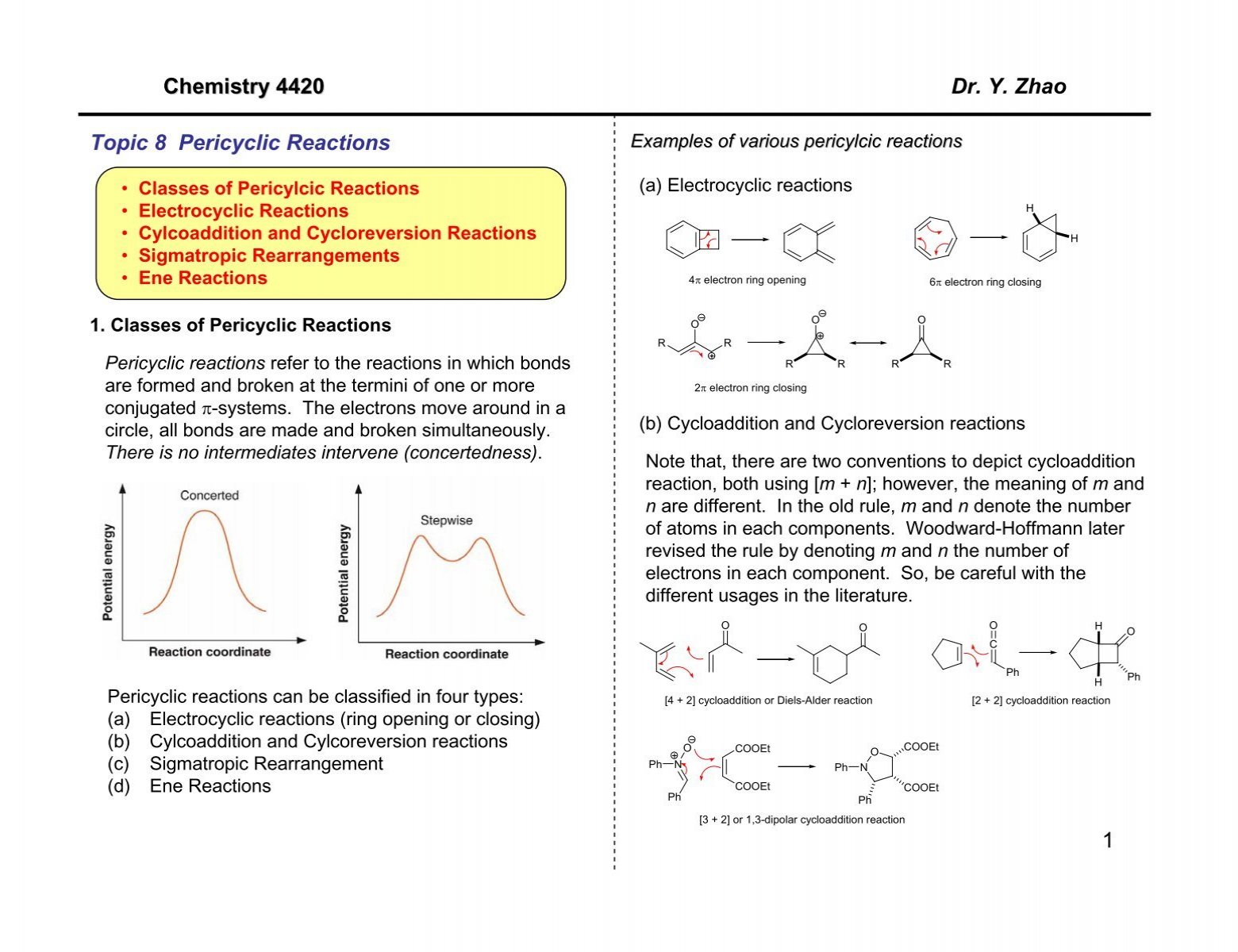



1 Chemistry 44 Dr Y Zhao Topic 8 Pericyclic Reactions
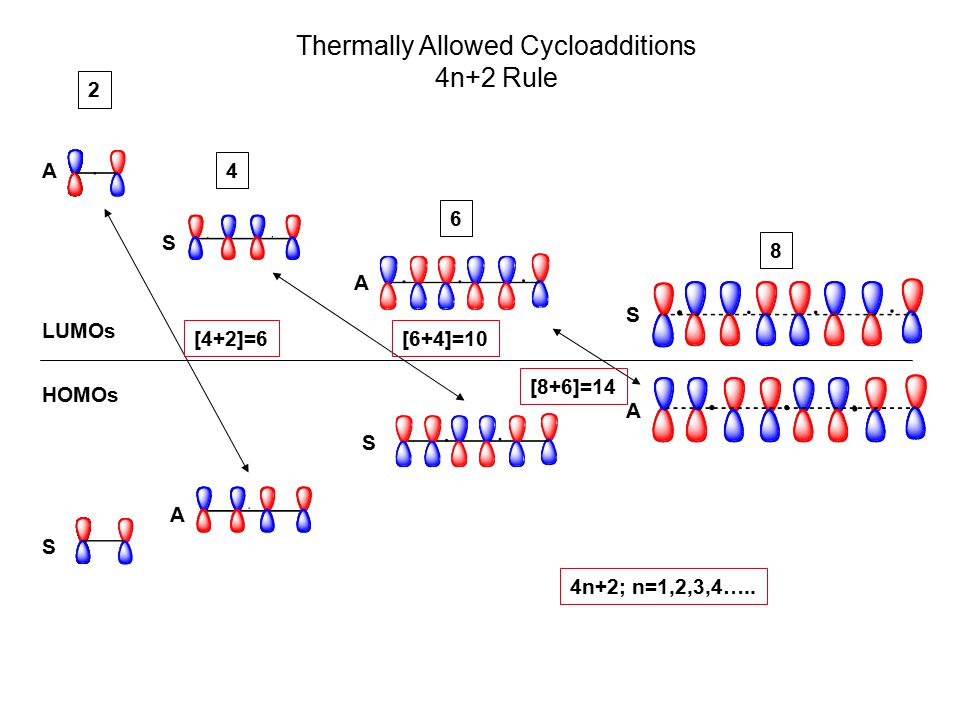



Title Pericyclic Reactions Cycloaddition Electrocyclization Ppt Video Online Download
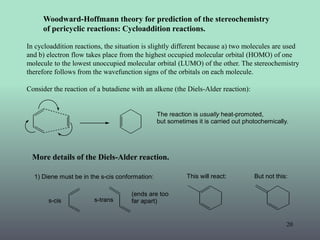



Pericyclic Reaction Woodward Hoffmann Rules Fmo Theory




Other Cycloadditions




Cycloaddition An Overview Sciencedirect Topics




How To Determine 4n And 4n 2 Electrocyclic Reactions Youtube




Cycloaddition Wikipedia
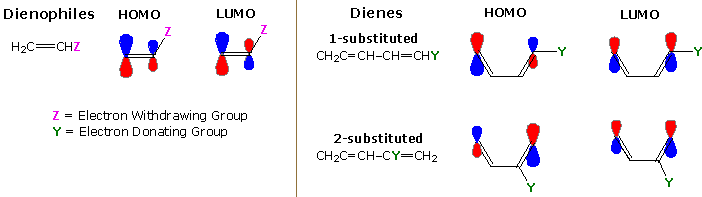



Non Ionic Chemical Reactions



Non Ionic Chemical Reactions




Organic Chemistry Application Of The Woodward Hoffman Rules To A 14 2 Cycloaddition Chemistry Stack Exchange




Other Cycloadditions



13 3 Cycloaddition Reactions Chemistry Libretexts




Woodward Hoffmann Rules Wikipedia




Cycloaddition Wikipedia



Examples Of Electrocyclic Reactions



Non Ionic Chemical Reactions



Cycloaddition




Quick Reference To Pericyclic Reactions And Photochemistry Claude Legault Litterature Meeting December 13 Th Ppt Download



Examples Of Cycloaddition Elimination Reactions




Overview Pages Perycyclic Book Pages 1 15 Flip Pdf Download Fliphtml5



2




Pericyclic Reaction An Overview Sciencedirect Topics
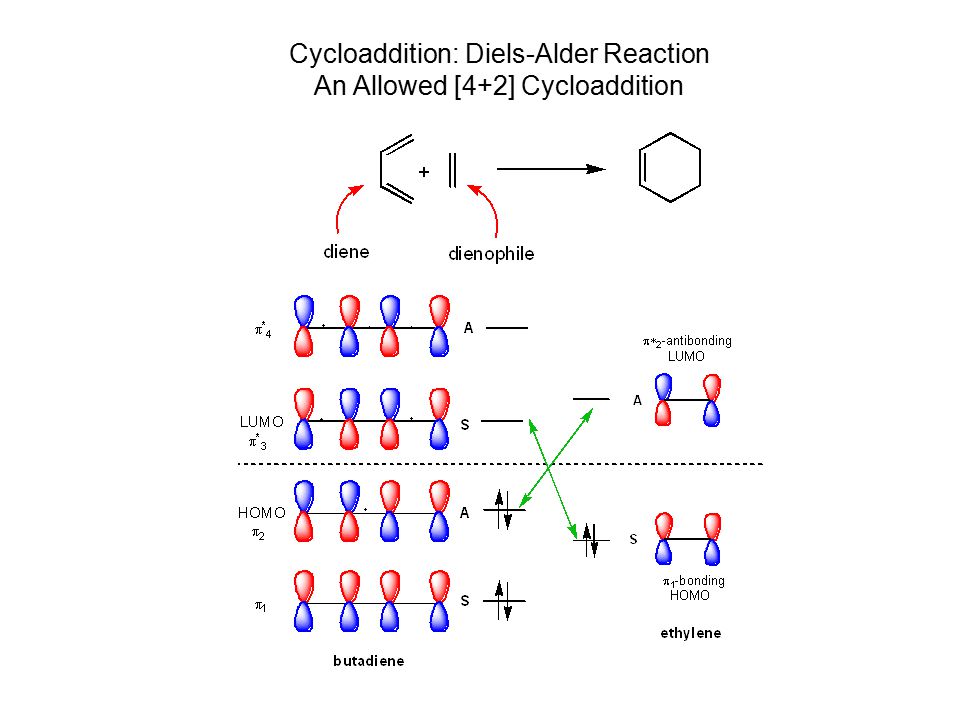



Title Pericyclic Reactions Cycloaddition Electrocyclization Ppt Video Online Download
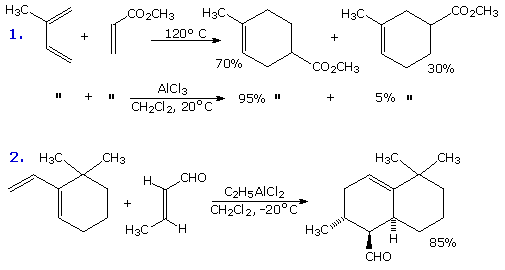



Non Ionic Chemical Reactions
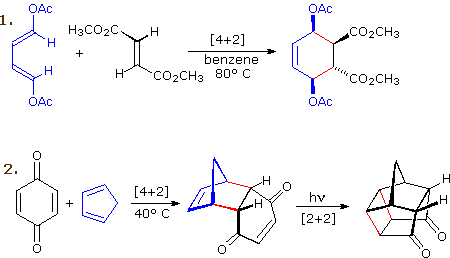



Non Ionic Chemical Reactions




Plausible Mechanism Of 3 Th 2 And 3 Th 3 Cycloaddition Download Scientific Diagram
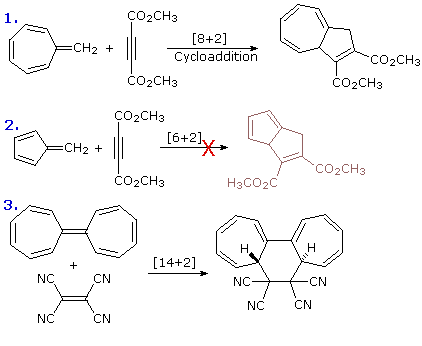



Non Ionic Chemical Reactions




Stereochemistry Of Electrocyclic Reactions Woodward Hoffmann Rules Youtube




Ak Lectures Summary Of Cycloaddition Reactions
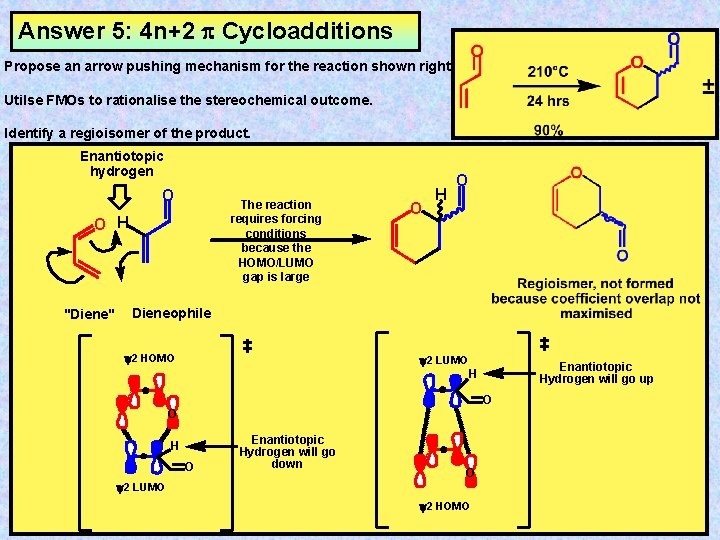



Cycloaddition Reactions Cycloaddition Reactions Are Intermolecular Pericyclic Processes
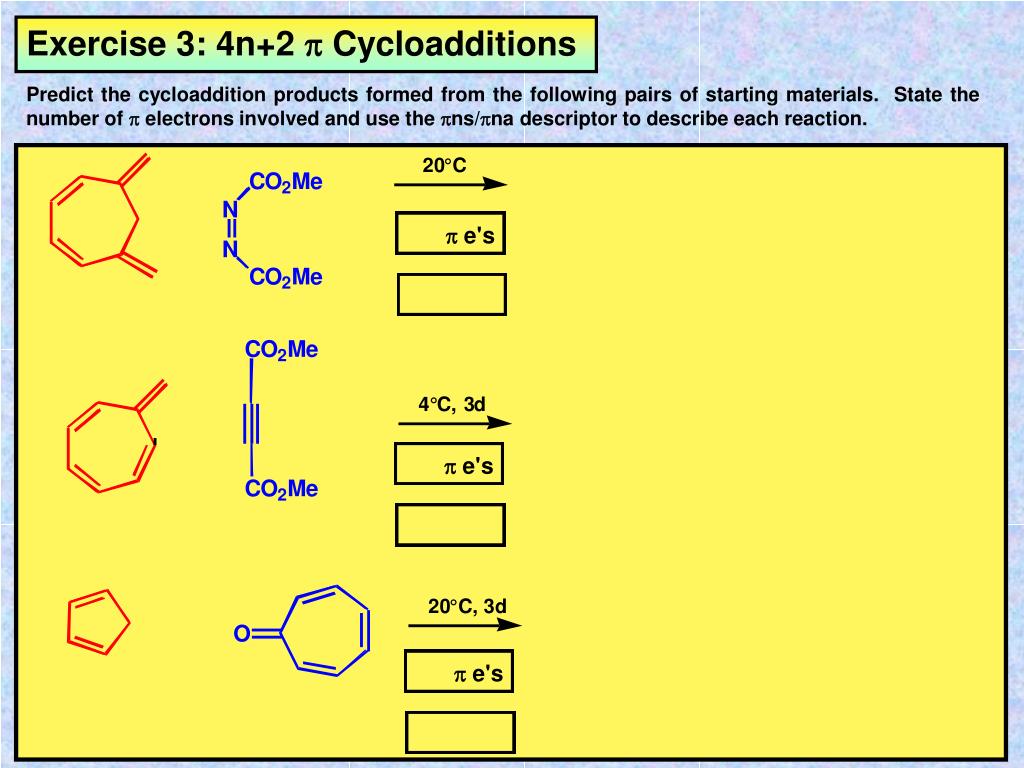



Ppt Third Year Organic Chemistry Course Chm3a2 Frontier Molecular Orbitals And Pericyclic Reactions Powerpoint Presentation Id




Electrocyclic Reactions




Woodward Hoffmann Rules Wikipedia



2
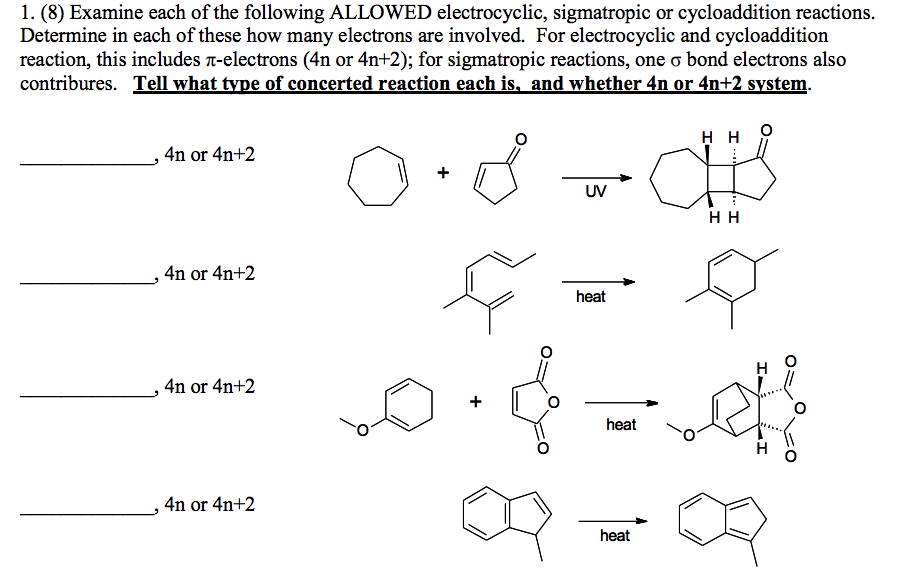



Solved 1 8 Examine Each Of The Following Allowed Chegg Com



0 件のコメント:
コメントを投稿